 |
Each long-acting, film-coated, dye-free, DURATUSS™ GP tablet for oral administration contains:
Guaifenesin ....................................................... 1200 mg
Pseudoephedrine hydrochloride ........................... 120 mg
Also contains microcrystalline cellulose, magnesium stearate and other ingredients. Film coating composed of hydroxypropyl methylcellulose and polyethylene glycol.
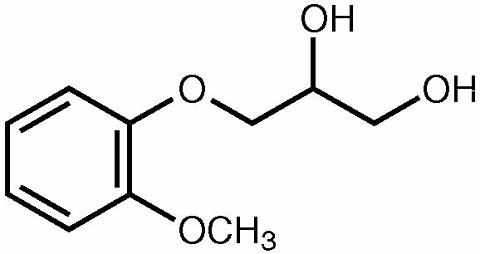
Guaifenesin is an expectorant with the chemical name 1,2-propanediol, 3-(2-methoxyphenoxy)-, (±)-. The molecular weight is 198.22. The molecular formula is C 10 H 14 O 4 . Guaifenesin occurs as white to slightly gray crystalline substance, which may have a slight characteristic odor. It is soluble in water, in alcohol, in chloroform, and in propylene glycol and sparingly soluble in glycerin. The chemical structure is shown below:
 |
Pseudoephedrine hydrochloride, is an adrenergic (vasoconstrictor) agent with the chemical name benzenemethanol, (alpha)-[1-(methylamino)etyl]-, [S-(R*,R*)]-, hydrochloride. The molecular weight is 201.69. The molecular formula is C 10 H 15 NO·HCl. Pseudoephedrine hydrochloride occurs as fine, white to off-white crystals or powder, having a faint characteristic odor. It is very soluble in water, freely soluble in alcohol and sparingly soluble in chloroform. The chemical structure is shown below:
 |
Overview: Guaifenesin is an expectorant, which increases respiratory tract fluid secretions and helps loosen phlegm and bronchial secretions. By reducing the viscosity of secretions, guaifenesin increases the efficiency of the mucociliary mechanism in removing accumulated secretions from the upper and lower airway. As a result, sinus and bronchial drainage is improved and nonproductive coughs become more productive and less frequent.
Pseudoephedrine hydrochloride is an orally effective nasal decongestant that acts on alpha-adrenergic receptors in the mucosa of the respiratory tract producing vasoconstriction. Pseudoephedrine shrinks swollen nasal mucus membranes, reduces tissue hyperemia, edema and nasal congestion and increases nasal airway patency. Drainage of sinus secretions is increased and obstructed eustachian ostia may be opened. Pseudoephedrine produces little if any rebound congestion. Pseudoephedrine produces peripheral effects similar to those of ephedrine and central effects similar to, but less intense than, amphetamines. It has potential for excitatory side effects.
Guaifenesin is readily absorbed from the gastrointestinal tract and is rapidly metabolized and excreted in the urine. Guaifenesin has a plasma half-life of one hour. The major urinary metabolite is (beta)-(2-methoxyphenoxy) lactic acid.
Pseudoephedrine has been shown to have a mean elimination half-life of 4-6 hours, which is dependent on urine pH. The elimination half-life is decreased at urine pH lower than 6 and may be increased at urine pH higher than 8.
Special Populations:
Pediatric Patients: DURATUSS GP contains a fixed dose of pseudoephedrine hydrochloride 120 mg in an extended release formulation. This product is not recommended for pediatric patients under 12 years of age.
Renal Impairment: About 55-75% of an administered dose of pseudoephedrine hydrochloride is excreted unchanged in the urine; the remainder is apparently metabolized in the liver. Therefore, pseudoephedrine may accumulate in patients with renal insufficiency.
Dosing adjustment may be necessary in patients with moderate or severe renal impairment and in patients on dialysis.
Hepatic Impairment: The effect of hepatic impairment on pseudoephedrine pharmacokinetics is unknown.
DURATUSS GP tablets are indicated for the relief of nasal congestion due to the common cold, hay fever or other upper respiratory allergies and nasal congestion associated with sinusitis. To promote nasal or sinus drainage; for the relief of eustachian tube congestion; for adjunctive therapy in serous otitis media; for the symptomatic relief of respiratory conditions characterized by dry, nonproductive cough and in the presence of tenacious mucus and/or mucus plugs in the respiratory tract.
DURATUSS GP is contraindicated in those patients with a known hypersensitivity to it or any of its ingredients.
Due to its pseudoephedrine component, DURATUSS GP is contraindicated in patients with narrow-angle glaucoma or urinary retention, and in patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment (see Drug/Drug Interactions ). It is also contraindicated in patients with severe hypertension, or severe coronary artery disease, and in those who have shown hypersensitivity, or idiosyncrasy to its components, to adrenergic agents, or to other drugs of similar chemical structures. Manifestations of patient idiosyncrasy to adrenergic agents include: insomnia, dizziness, weakness, tremor, or arrhythmias.
Sympathomimetic amines should be used judiciously and sparingly in patients with hypertension, peripheral vascular disease, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism, renal impairment, or prostatic hypertrophy (see CONTRAINDICATIONS ). Sympathomimetic amines may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension. The elderly are more likely to have adverse reactions to sympathomimetic amines.
Information for Patients: Patients taking DURATUSS GP should receive the following information: Patients should be instructed to take DURATUSS GP only as prescribed. Do not exceed the recommended dose. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult physician.
Patients who are hypersensitive to it or any of its ingredients should not use this product. Due to its pseudoephedrine component, this product should not be used by patients with narrow-angle glaucoma, urinary retention, or patients receiving a monoamine oxidase (MAO) inhibitor or within 14 days of stopping use of a MAO inhibitor. Patients with severe hypertension or severe coronary artery disease also should not use it.
As with other sympathomimetic drugs, DURATUSS GP should be used cautiously in the presence of hypertension, peripheral vascular disease, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism, renal impairment, or prostatic hypertrophy. Sympathomimetic amines may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension. The elderly are more likely to have adverse reactions to sympathomimetic amines.
Patients should consult their physician if they are or wish to become pregnant.
Do not crush or chew DURATUSS GP tablets before ingestion to preserve the long-acting effect.
Drug/Drug Interactions: Due to the pseudoephedrine component, DURATUSS GP is contraindicated in patients taking monoamine oxidase (MAO) inhibitors and for fourteen (14) days after stopping use of a MAO inhibitor. Concomitant use with antihypertensive drugs which interfere with sympathetic activity (e.g. methyldopa, mecamylamine, and reserpine) may reduce their antihypertensive effects. Increased ectopic pacemaker activity can occur when pseudoephedrine is used concomitantly with digitalis. Care should be taken in the administration of DURATUSS GP concomitantly with other sympathomimetic amines because combined effects on the cardiovascular system may be harmful to the patient (See CONTRAINDICATIONS and ).
Drug/Laboratory Test Interactions: Guaifenesin interferes with the colorimetric determination of 5-hydroxy-indolacetic acid (5-HIAA) and vanillylmandelic acid (VMA).
Carcinogenesis, Mutagenesis, Impairment of Fertility: There are no animal or in vitro studies on the combination product guaifenesin and pseudoephedrine hydrochloride to evaluate carcinogenesis, mutagenesis, and impairment of fertility.
Two-year feeding studies in rats and mice conducted under the auspices of National Toxicology Program (NTP) demonstrated no evidence of carcinogenic potential with ephedrine sulfate, a structurally related drug with pharmacological properties similar to pseudoephedrine, at doses up to 10 and 27 mg/kg, respectively (approximately 16 and 100% of the maximum recommended daily dose of pseudoephedrine hydrochloride in adults on a mg/m 2 basis
Pregnancy Category C: There are no adequate and well-controlled studies with guaifenesin and/or pseudoephedrine hydrochloride in pregnant women. DURATUSS GP should be used in pregnant women only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: It is not known if guaifenesin is excreted in human milk.
Pseudoephedrine hydrochloride administered alone distributes into breast milk of lactating human females.
Because of the potential for serious adverse reactions in nursing infants from sympathomimetic amines, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: DURATUSS GP contains a fixed dose of pseudoephedrine hydrochloride 120 mg in an extended release formulation. This product is not recommended for pediatric patients under 12 years of age. The safety and effectiveness of DURATUSS GP in pediatric patients under the age of 12 years have not been established.
Geriatric Use: The elderly are more likely to have adverse reactions to sympathomimetic amines. In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. The pseudoephedrine component of DURATUSS GP is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Use in Patients with Impaired Renal Function: Dose should be adjusted in patients with decreased renal function. Patients should be given a lower total daily dose because they have reduced elimination of pseudoephedrine.
Guaifenesin Guaifenesin is well tolerated. Side effects are generally mild and infrequent. Nausea and vomiting are the most frequently occurring side effects. Dizziness, headache, and rash (including urticaria) have been reported rarely.
Pseudoephedrine hydrochloride: Pseudoephedrine hydrochloride may cause mild CNS stimulation in hypersensitivity patients. Nervousness, excitability, dizziness, weakness, or insomnia may occur. Headache, nausea, drowsiness, tachycardia, palpitation, pressor activity, and cardiac arrhythmias have been reported. Sympathomimetic drugs have also been associated with other untoward effects such as fear, anxiety, tenseness, tremor, hallucinations, seizures, pallor, respiratory difficulty, dysuria, and cardiovascular collapse.
There is no information to indicate that abuse or dependency occurs with guaifenesin or with the combination of guaifenesin and pseudoephedrine.
However, like other central nervous system stimulants, pseudoephedrine has been abused. At high doses, subjects commonly experience an elevation of mood; a sense of increased energy and alertness and decreased appetite. Some individuals become anxious, irritable, and loquacious. In addition to the marked euphoria, the user experiences a sense of markedly enhanced physical strength and mental capacity. With continued use, tolerance develops, the user increases the dose, and toxic signs and symptoms appear. Depression may follow rapid withdrawal.
Since the effects of DURATUSS GP may last up to 12 hours, treatment of overdosage should be directed towards supporting the patient and reversing the effects of the drug for at least that length of time.
In large doses, sympathomimetics may give rise to giddiness, headache, nausea, vomiting, sweating, thirst, tachycardia, precordial pain, palpitations, difficulty in micturition, muscular weakness and tenseness, anxiety, restlessness, and insomnia. Many patients can present a toxic psychosis with delusions and hallucinations. Some may develop cardiac arrhythmias, circulatory collapse, convulsions, coma and respiratory failure.
Adults and pediatric patients 12 years of age and older: The recommended dose of DURATUSS GP is one tablet every 12 hours, not to exceed 2 tablets in 24 hours.
Dosing adjustments may be necessary in patients with moderate or severe renal impairment and in patients on dialysis.
Do not crush or chew DURATUSS GP tablets before ingestion to preserve the long-acting effect.
DURATUSS™ GP (1200 mg guaifenesin and 120 mg pseudoephedrine hydrochloride) is supplied as scored, white, film-coated, dye-free, oval-shaped tablets, debossed "ucb/640" on one side, in bottles of 100 tablets, NDC 50474-640-01.
Store at controlled room temperature 25°C (77°F); excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature]
Protect from light and moisture.
Manufactured for
UCB Pharma, Inc.
Smyrna, GA 30080
Manufactured by
Mikart, Inc.
Atlanta, GA 30318
Rev. 5/00
P/N: 1003322
Code 0870A00
 |