 |
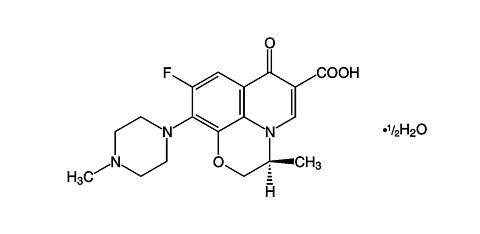
LEVAQUIN® (levofloxacin tablets/injection) Tablets/Injection are synthetic broad spectrum antibacterial agents for oral and intravenous administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug substance ofloxacin. The chemical name is (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)- 7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate.
 |
Its empirical formula is C 18 H 20 FN 3 O 4 · 1 / 2 H 2 O and its molecular weight is 370.38. Levofloxacin is a light yellowish-white to yellow-white crystal or crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine.
The data demonstrate that from pH 0.6 to 5.8, the solubility of levofloxacin is essentially constant (approximately 100 mg/mL). Levofloxacin is considered soluble to freely soluble in this pH range, as defined by USP nomenclature. Above pH 5.8, the solubility increases rapidly to its maximum at pH 6.7 (272 mg/mL) and is considered freely soluble in this range. Above pH 6.7, the solubility decreases and reaches a minimum value (about 50 mg/mL) at a pH of approximately 6.9.
Levofloxacin has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Al +3 >Cu +2 >Zn +2 >Mg +2 >Ca +2 .
LEVAQUIN Tablets are available as film-coated tablets and contain the following inactive ingredients: 250-mg (as expressed in the anhydrous form): hydroxypropyl methylcellulose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80 and synthetic red iron oxide.
500-mg (as expressed in the anhydrous form): hydroxypropyl methylcellulose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80 and synthetic red and yellow iron oxides.
LEVAQUIN Injection in Single-Use Vials is a sterile, preservative-free aqueous solution of levofloxacin with pH ranging from 3.8 to 5.8. LEVAQUIN Injection in Premix Flexible Containers is a sterile, preservative-free aqueous solution of levofloxacin with pH ranging from 3.8 to 5.8. The appearance of LEVAQUIN Injection may range from a clear yellow to a greenish-yellow solution. This does not adversely affect product potency.
LEVAQUIN Injection in Single-Use Vials contains levofloxacin in Water for Injection. LEVAQUIN Injection in Premix Flexible Containers is a dilute, non-pyrogenic, nearly isotonic premixed solution that contains levofloxacin in 5% Dextrose (D 5 W). Solutions of hydrochloric acid and sodium hydroxide may have been added to adjust the pH.
The flexible container is fabricated from a specially formulated non-plasticized, thermoplastic copolyester (CR3). The amount of water that can permeate from the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the flexible container can leach out certain of the container's chemical components in very small amounts within the expiration period. The suitability of the container material has been confirmed by tests in animals according to USP biological tests for plastic containers.
Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained one to two hours after oral dosing. Following a single 60-minute intravenous infusion of 500-mg of levofloxacin to healthy volunteers, the mean peak plasma concentration attained was 6.2 µg/mL. The absolute bioavailability of a 500-mg oral dose of levofloxacin is approximately 99%.
Levofloxacin pharmacokinetics are linear and predictable after single and multiple oral and i.v. dosing regimens. Steady-state is reached within 48 hours following a 500-mg once-daily regimen. The peak and trough plasma concentrations attained following multiple once-daily oral 500-mg regimens were approximately 5.7 and 0.5 µg/mL, respectively. The peak and trough plasma concentrations attained following multiple once-daily i.v. 500-mg regimens were approximately 6.4 and 0.6 µg/mL, respectively.
Oral administration with food slightly prolongs the time to peak concentration by approximately 1 hour and slightly decreases the peak concentration by approximately 14%. Therefore, levofloxacin can be administered without regard to food.
The plasma concentration profile of levofloxacin after i.v. administration is similar and comparable in extent of exposure (AUC) to that observed for levofloxacin tablets when equal doses (mg/mg) are administered. Therefore, the oral and i.v. routes of administration can be considered interchangeable. (See following chart.)
 |
The mean volume of distribution of levofloxacin generally ranges from 89 to 112 L after single and multiple 500-mg doses, indicating widespread distribution into body tissues. Penetration of levofloxacin into blister fluid is rapid and extensive. The blister fluid to plasma AUC ratio is approximately 1. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2- to 5- fold higher than plasma concentrations and ranged from approximately 2.4 to 11.3 µg/g over a 24-hour period after a single 500-mg oral dose.
In vitro , over a clinically relevant range (1 to 10 µg/mL) of serum/plasma levofloxacin concentrations, levofloxacin is approximately 24 to 38% bound to serum proteins across all species studied, as determined by the equilibrium dialysis method. Levofloxacin is mainly bound to serum albumin in humans. Levofloxacin binding to serum proteins is independent of the drug concentration.
Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered as unchanged drug in urine within 48 hours, whereas less than 4% of the dose was recovered in feces in 72 hours. Less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6 to 8 hours following single or multiple doses of levofloxacin given orally or intravenously. The mean apparent total body clearance and renal clearance range from approximately 144 to 226 mL/min and 96 to 142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid results in approximately 24% and 35% reduction in the levofloxacin renal clearance, respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving levofloxacin.
Geriatric There are no significant differences in levofloxacin pharmacokinetics between young and elderly subjects when the subjects' differences in creatinine clearance are taken into consideration. Following a 500-mg oral dose of levofloxacin to healthy elderly subjects (66-80 years of age), the mean terminal plasma elimination half-life of levofloxacin was about 7.6 hours, as compared to approximately 6 hours in younger adults. The difference was attributable to the variation in renal function status of the subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by age. Levofloxacin dose adjustment based on age alone is not necessary.
Pediatric The pharmacokinetics of levofloxacin in pediatric subjects have not been studied.
Gender There are no significant differences in levofloxacin pharmacokinetics between male and female subjects when subjects' differences in creatinine clearance are taken into consideration. Following a 500-mg oral dose of levofloxacin to healthy male subjects, the mean terminal plasma elimination half-life of levofloxacin was about 7.5 hours, as compared to approximately 6.1 hours in female subjects. This difference was attributable to the variation in renal function status of the male and female subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by the gender of the subjects. Dose adjustment based on gender alone is not necessary.
Race: The effect of race on levofloxacin pharmacokinetics was examined through a covariate analysis performed on data from 72 subjects: 48 white and 24 nonwhite. The apparent total body clearance and apparent volume of distribution were not affected by the race of the subjects.
Renal insufficiency: Clearance of levofloxacin is reduced and plasma elimination half-life is prolonged in patients with impaired renal function (creatinine clearance </=80 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of levofloxacin are not required following hemodialysis or CAPD. (See PRECAUTIONS: General and DOSAGE AND ADMINISTRATION .)
Hepatic insufficiency: Pharmacokinetic studies in hepatically impaired patients have not been conducted. Due to the limited extent of levofloxacin metabolism, the pharmacokinetics of levofloxacin are not expected to be affected by hepatic impairment.
Bacterial infection: The pharmacokinetics of levofloxacin in patients with serious community-acquired bacterial infections are comparable to those observed in healthy subjects.
Drug-drug interactions: The potential for pharmacokinetic drug interactions between levofloxacin and theophylline, warfarin, cyclosporine, digoxin, probenecid, cimetidine, sucralfate, and antacids has been evaluated. (See PRECAUTIONS : Drug Interactions .)
The mean (± SD) pharmacokinetic parameters of levofloxacin determined under single and steady state conditions following oral (p.o.) or intravenous (i.v.) doses of levofloxacin are summarized as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Levofloxacin is the L-isomer of the racemate, ofloxacin, a quinolone antimicrobial agent. The antibacterial activity of ofloxacin resides primarily in the L-isomer. The mechanism of action of levofloxacin and other fluoroquinolone antimicrobials involves inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair and recombination.
Levofloxacin has in vitro activity against a wide range of gram-negative and gram-positive microorganisms. Levofloxacin is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Fluoroquinolones, including levofloxacin, differ in chemical structure and mode of action from aminoglycosides, macrolides and (beta)-lactam antibiotics, including penicillins. Fluoroquinolones may, therefore, be active against bacteria resistant to these antimicrobials.
Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10 -9 to 10 -10 ). Although cross-resistance has been observed between levofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to levofloxacin.
Levofloxacin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the section:
Aerobic gram-positive microorganisms
Enterococcus faecalis (many strains are only moderately susceptible)
Staphylococcus aureus (methicillin-susceptible strains)
Staphylococcus saprophyticus
Streptococcus pneumoniae (including penicillin-resistant strains * )
Streptococcus pyogenes
*Note: penicillin-resistant S. pneumoniae are those strains with a penicillin MIC value of >/=2 µg/mL
Aerobic gram-negative microorganisms
Enterobacter cloacae
Escherichia coli
Haemophilus influenzae
Haemophilus parainfluenzae
Klebsiella pneumoniae
Legionella pneumophila
Moraxella catarrhalis
Proteus mirabilis
Pseudomonas aeruginosa
As with other drugs in this class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with levofloxacin.
Other microorganisms
Chlamydia pneumoniae
Mycoplasma pneumoniae
The following in vitro data are available, but their clinical significance is unknown .
Levofloxacin exhibits in vitro minimum inhibitory concentrations (MIC values) of 2 µg/mL or less against most (>/= 90%) strains of the following microorganisms; however, the safety and effectiveness of levofloxacin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.
Aerobic gram-positive microorganisms
Staphylococcus epidermidis
(methicillin-susceptible strains)
Streptococcus (Group C/F)
Streptococcus (Group G)
Streptococcus agalactiae
Viridans group streptococci
Aerobic gram-negative microorganisms
Acinetobacter baumannii
Acinetobacter calcoaceticus
Acinetobacter lwoffii
Bordetella pertussis
Citrobacter (diversus) koseri
Citrobacter freundii
Enterobacter aerogenes
Enterobacter sakazakii
Klebsiella oxytoca
Morganella morganii
Pantoea (Enterobacter) agglomerans
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Pseudomonas fluorescens
Serratia marcescens
Anaerobic gram-positive microorganisms
Clostridium perfringens
Susceptibility testing for levofloxacin should be performed, as it is the optimal predictor of activity.
Dilution techniques: Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MIC values). These MIC values provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC values should be determined using a standardized procedure. Standardized procedures are based on a dilution method 1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of levofloxacin powder. The MIC values should be interpreted according to the following criteria:
For testing aerobic microorganisms other than Haemophilus influenzae, Haemophilus parainfluenzae, and Streptococcus spp. including S. pneumoniae:
|
|
||||||
The current absence of data on resistant strains precludes defining any categories other than "Susceptible". Strains yielding MIC results suggestive of a "nonsusceptible" category should be submitted to a reference laboratory for further testing.
|
||||||||||
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard levofloxacin powder should give the following MIC values:
|
||||||||||||||||||||||||||||||
Diffusion techniques: Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure 2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 5-µg levofloxacin to test the susceptibility of microorganisms to levofloxacin.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 5-µg levofloxacin disk should be interpreted according to the following criteria:
For aerobic microorganisms other than Haemophilus influenzae, Haemophilus parainfluenzae, and Streptococcus spp. including S. pneumoniae:
|
|
||||||
The current absence of data on resistant strains precludes defining any categories other than "Susceptible". Strains yielding zone diameter results suggestive of a "nonsusceptible" category should be submitted to a reference laboratory for further testing.
|
||||||||||
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for levofloxacin.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. For the diffusion technique, the 5-µg levofloxacin disk should provide the following zone diameters in these laboratory test quality control strains:
|
||||||||||||||||||||||||
LEVAQUIN Tablets/Injection are indicated for the treatment of adults (>/= 18 years of age) with mild, moderate, and severe infections caused by susceptible strains of the designated microorganisms in the conditions listed below. LEVAQUIN Injection is indicated when intravenous administration offers a route of administration advantageous to the patient (e.g., patient cannot tolerate an oral dosage form). Please see DOSAGE AND ADMINISTRATION for specific recommendations.
Acute maxillary sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae , or Moraxella catarrhalis.
Acute bacterial exacerbation of chronic bronchitis due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis .
Community-acquired pneumonia due to Staphylococcus aureus, Streptococcus pneumoniae (including penicillin-resistant strains, MIC value for penicillin >/=2 µg/mL), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydia pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae . (See CLINICAL STUDIES .)
Uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections, due to Staphylococcus aureus , or Streptococcus pyogenes .
Complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa .
Acute pyelonephritis (mild to moderate) caused by Escherichia coli .
Uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin. Therapy with levofloxacin may be initiated before results of these tests are known; once results become available, appropriate therapy should be selected.
As with other drugs in this class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with levofloxacin. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance.
Levofloxacin is contraindicated in persons with a history of hypersensitivity to levofloxacin, quinolone antimicrobial agents, or any other components of this product.
THE SAFETY AND EFFICACY OF LEVOFLOXACIN IN PEDIATRIC PATIENTS, ADOLESCENTS (UNDER THE AGE OF 18 YEARS), PREGNANT WOMEN, AND NURSING WOMEN HAVE NOT BEEN ESTABLISHED . (See PRECAUTIONS : Pediatric Use Pregnancy and Nursing Mothers subsections.)
In immature rats and dogs, the oral and intravenous administration of levofloxacin increased the incidence and severity of osteochondrosis. Other fluoroquinolones also produce similar erosions in the weight bearing joints and other signs of arthropathy in immature animals of various species. (See ANIMAL PHARMACOLOGY .)
Convulsions and toxic psychoses have been reported in patients receiving quinolones, including levofloxacin. Quinolones may also cause increased intracranial pressure and central nervous system stimulation which may lead to tremors, restlessness, anxiety, lightheadedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and, rarely, suicidal thoughts or acts. These reactions may occur following the first dose. If these reactions occur in patients receiving levofloxacin, the drug should be discontinued and appropriate measures instituted. As with other quinolones, levofloxacin should be used with caution in patients with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction.) (See PRECAUTIONS: General, Information for Patients, Drug Interactions and ADVERSE REACTIONS .)
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with quinolones, including levofloxacin. These reactions often occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat, or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath, and acute respiratory distress), dyspnea, urticaria, itching, and other serious skin reactions. Levofloxacin should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated. (See PRECAUTIONS and ADVERSE REACTIONS .)
Serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including levofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following: fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome); vasculitis; arthralgia; myalgia; serum sickness; allergic pneumonitis; interstitial nephritis; acute renal insufficiency or failure; hepatitis; jaundice; acute hepatic necrosis or failure; anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities. The drug should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity and supportive measures instituted. (See PRECAUTIONS: Information for Patients and ADVERSE REACTIONS .)
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including levofloxacin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agent.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of "antibiotic-associated colitis."
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis. (See ADVERSE REACTIONS .)
Ruptures of the shoulder, hand, or Achilles tendons that required surgical repair or resulted in prolonged disability have been reported in patients receiving quinolones, including levofloxacin. Levofloxacin should be discontinued if the patient experiences pain, inflammation, or rupture of a tendon. Patients should rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded. Tendon rupture can occur during or after therapy with quinolones, including levofloxacin.
Because a rapid or bolus intravenous injection may result in hypotension, LEVOFLOXACIN INJECTION SHOULD ONLY BE ADMINISTERED BY SLOW INTRAVENOUS INFUSION OVER A PERIOD OF 60 MINUTES. (See DOSAGE AND ADMINISTRATION .)
Although levofloxacin is more soluble than other quinolones, adequate hydration of patients receiving levofloxacin should be maintained to prevent the formation of a highly concentrated urine.
Administer levofloxacin with caution in the presence of renal insufficiency. Careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of levofloxacin may be reduced. In patients with impaired renal function (creatinine clearance </=80 mL/min), adjustment of the dosage regimen is necessary to avoid the accumulation of levofloxacin due to decreased clearance. (See CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION .)
Moderate to severe phototoxicity reactions have been observed in patients exposed to direct sunlight while receiving drugs in this class. Excessive exposure to sunlight should be avoided. However, in clinical trials with levofloxacin, phototoxicity has been observed in less than 0.1% of patients. Therapy should be discontinued if phototoxicity (e.g., a skin eruption) occurs.
As with other quinolones, levofloxacin should be used with caution in any patient with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction). (See and Drug Interactions .)
As with other quinolones, disturbances of blood glucose, including symptomatic hyper- and hypoglycemia, have been reported, usually in diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (e.g., glyburide/glibenclamide) or with insulin. In these patients, careful monitoring of blood glucose is recommended. If a hypoglycemic reaction occurs in a patient being treated with levofloxacin, levofloxacin should be discontinued immediately and appropriate therapy should be initiated immediately. (See Drug Interactions and ADVERSE REACTIONS .)
Some quinolones have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. During post-marketing surveillance, extremely rare cases of torsades de pointes, have been reported in patients taking levofloxacin. These reports generally involve patients who had other concurrent medical conditions and the relationship to levofloxacin has not been established. Among drugs known to cause prolongation of the QT interval, the risk of arrhythmias may be reduced by avoiding use in the presence of hypokalemia, significant bradycardia, or concurrent treatment with class Ia or class III antiarrhythmic agents.
As with any potent antimicrobial drug, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during therapy. (See and ADVERSE REACTIONS .)
Patients should be advised:
LEVAQUIN Tablets: While the chelation by divalent cations is less marked than with other quinolones, concurrent administration of LEVAQUIN Tablets with antacids containing magnesium, or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc may interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. Tablets with antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamins preparations with zinc or Videx®, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution may substantially interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. These agents should be taken at least two hours before or two hours after levofloxacin administration.
LEVAQUIN Injection: There are no data concerning an interaction of Intravenous quinolones with oral antacids, sucralfate, multivitamins, Videx®, (Didanosine), or metal cations. However, no quinolone should be co-administered with any solution containing multivalent cations, e.g., magnesium, through the same intravenous line. (See DOSAGE AND ADMINISTRATION .)
Theophylline No significant effect of levofloxacin on the plasma concentrations, AUC, and other disposition parameters for theophylline was detected in a clinical study involving 14 healthy volunteers. Similarly, no apparent effect of theophylline on levofloxacin absorption and disposition was observed. However, concomitant administration of other quinolones with theophylline has resulted in prolonged elimination half-life, elevated serum theophylline levels, and a subsequent increase in the risk of theophylline-related adverse reactions in the patient population. Therefore, theophylline levels should be closely monitored and appropriate dosage adjustments made when levofloxacin is co-administered. Adverse reactions, including seizures, may occur with or without an elevation in serum theophylline levels. (See and PRECAUTIONS: General .)
Warfarin: No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for R- and S- warfarin was detected in a clinical study involving healthy volunteers. Similarly, no apparent effect of warfarin on levofloxacin absorption and disposition was observed. There have been reports during the post-marketing experience in patients that levofloxacin enhances the effects of warfarin. Elevations of the prothrombin time in the setting of concurrent warfarin and levofloxacin use have been associated with episodes of bleeding. Prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored if levofloxacin is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding.
Cyclosporine: No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for cyclosporine was detected in a clinical study involving healthy volunteers. However, elevated serum levels of cyclosporine have been reported in the patient population when co-administered with some other quinolones. Levofloxacin C max and k e were slightly lower while T max and t 1/2 were slightly longer in the presence of cyclosporine than those observed in other studies without concomitant medication. The differences, however, are not considered to be clinically significant. Therefore, no dosage adjustment is required for levofloxacin or cyclosporine when administered concomitantly.
Digoxin No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for digoxin was detected in a clinical study involving healthy volunteers. Levofloxacin absorption and disposition kinetics were similar in the presence or absence of digoxin. Therefore, no dosage adjustment for levofloxacin or digoxin is required when administered concomitantly.
Probenecid and Cimetidine: No significant effect of probenecid or cimetidine on the rate and extent of levofloxacin absorption was observed in a clinical study involving healthy volunteers. The AUC and t 1/2 of levofloxacin were 27-38% and 30% higher, respectively, while CL/F and CL R were 21-35% lower during concomitant treatment with probenecid or cimetidine compared to levofloxacin alone. Although these differences were statistically significant, the changes were not high enough to warrant dosage adjustment for levofloxacin when probenecid or cimetidine is co-administered.
Non-steroidal anti-inflammatory drugs: The concomitant administration of a non-steroidal anti-inflammatory drug with a quinolone, including levofloxacin, may increase the risk of CNS stimulation and convulsive seizures. (See and PRECAUTIONS: General .)
Antidiabetic agents: Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with quinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered.
In a long term carcinogenicity study in rats, levofloxacin exhibited no carcinogenic or tumorigenic potential following daily dietary administration for 2 years; the highest dose was 2 or 10 times the recommended human dose based on surface area or body weight, respectively.
Levofloxacin was not mutagenic in the following assays; Ames bacterial mutation assay ( S. typhimurium and E. coli ), CHO/HGPRT forward mutation assay, mouse micronucleus test, mouse dominant lethal test, rat unscheduled DNA synthesis assay, and the mouse sister chromatid exchange assay. It was positive in the in vitro chromosomal aberration (CHL cell line) and sister chromatid exchange (CHL/IU cell line) assays.
Levofloxacin caused no impairment of fertility or reproductive performance in rats at oral doses as high as 360 mg/kg/day (2124 mg/m 2 ), corresponding to 3.0 or 18 times the recommended maximum human dose based on surface area or body weight, respectively, and intravenous doses as high as 100 mg/kg/day (590 mg/m 2 ), corresponding to 1.0 or 5 times the recommended maximum human dose based on surface area or body weight, respectively.
Levofloxacin was not teratogenic in rats at oral doses as high as 810 mg/kg/day (4779 mg/m 2 ), which corresponds to 14 or 82 times the recommended maximum human dose based on surface area or body weight, respectively, or at intravenous doses as high as 160 mg/kg/day (944 mg/m 2 ) corresponding to 2.7 or 16 times the recommended maximum human dose based on surface area or body weight, respectively. Doses equivalent to 26 or 81 times the recommended maximum human dose of levofloxacin (based on surface area or body weight, respectively) caused decreased fetal body weight and increased fetal mortality in rats when administered orally at 810 mg/kg/day (8910 mg/m 2 ). No teratogenicity was observed when rabbits were dosed orally as high as 50 mg/kg/day (550 mg/m 2 ) which corresponds to 1.6 or 5.0 times the recommended maximum human dose based on surface area or body weight, respectively, or when dosed intravenously as high as 25 mg/kg/day (275 mg/m 2 ), corresponding to 0.8 or 2.5 times the maximum recommended human dose based on surface area or body weight, respectively.
There are, however, no adequate and well-controlled studies in pregnant women. Levofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (See .)
Levofloxacin has not been measured in human milk. Based upon data from ofloxacin, it can be presumed that levofloxacin will be excreted in human milk. Because of the potential for serious adverse reactions from levofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Safety and effectiveness in pediatric patients and adolescents below the age of 18 years have not been established. Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species. (See .)
In phase 3 clinical trials, 898 levofloxacin-treated patients (26%) were >/=65 years of age. Of these, 514 patients (15%) were between the ages of 65 and 74 and 384 patients (11%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
The pharmacokinetic properties of levofloxacin in younger adults and elderly adults do not differ significantly when creatinine clearance is taken into consideration. However since the drug is known to be substantially excreted by the kidney, the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
The incidence of drug-related adverse reactions in patients during Phase 3 clinical trials conducted in North America was 6.2%. Among patients receiving levofloxacin therapy, 3.4% discontinued levofloxacin therapy due to adverse experiences.
In clinical trials, the following events were considered likely to be drug-related in patients receiving levofloxacin: nausea 1.3%, diarrhea 1.1%, vaginitis 0.7%, pruritus 0.5%, abdominal pain 0.4%, dizziness 0.4%, flatulence 0.4%, rash 0.4%, dyspepsia 0.3%, genital moniliasis 0.3%, insomnia 0.3%, taste perversion 0.2%, vomiting 0.2%, anorexia 0.1%, anxiety 0.1%, constipation 0.1%, edema 0.1%, fatigue 0.1%, fungal infection 0.1%, headache 0.1%, increased sweating 0.1%, leukorrhea 0.1%, malaise 0.1%, nervousness 0.1%, sleep disorders 0.1%, tremor 0.1%, urticaria 0.1%.
In clinical trials, the following events occurred in >3% of patients, regardless of drug relationship: nausea 7.1%, headache 6.4%, diarrhea 5.6%, injection site reaction 5.6%, insomnia 4.0%.
In clinical trials, the following events occurred in 1 to 3% of patients, regardless of drug relationship: constipation 2.9%, dizziness 2.9%, injection site pain 2.7%, abdominal pain 2.6%, dyspepsia 2.5%, vomiting 2.2%, rash 1.7%, flatulence 1.6%, vaginitis 1.6%, injection site inflammation 1.5%, pruritus 1.5%, fatigue 1.3%, back pain 1.2%, pain 1.2%, chest pain 1.1%, pharyngitis 1.1%, rhinitis 1.1%, taste perversion 1.0%.
In clinical trials, the following events occurred in 0.5 to less than 1% of patients, regardless of drug relationship: anorexia, anxiety, arthralgia, coughing, dry mouth, dyspnea, ear disorder (not otherwise specified), edema, fever, fungal infection, genital pruritus, increased sweating, skin disorder, somnolence.
In clinical trials, the following events, of potential medical importance, occurred at a rate of less than 0.5% regardless of drug relationship: abnormal coordination, abnormal dreaming, abnormal hepatic function, abnormal platelets, abnormal renal function, abnormal vision, acute renal failure, aggravated diabetes mellitus, aggressive reaction, agitation, anemia, angina pectoris, ARDS, arrhythmia, arthritis, arthrosis, asthenia, asthma, atrial fibrillation, bradycardia, cardiac arrest, cardiac failure, carcinoma, cerebrovascular disorder, cholelithiasis, circulatory failure, coma, confusion, conjunctivitis, convulsions (seizures), coronary thrombosis, dehydration, delirium, depression, diplopia, dysphagia, ejaculation failure, embolism (blood clot), emotional lability, epistaxis, erythema nodosum, face edema, gastroenteritis, genital moniliasis, G.I. hemorrhage, granulocytopenia, haematuria, haemoptysis, hallucination, heart block, hepatic coma, hyperglycemia, hyperkalemia, hyperkinesia, hypertension, hypertonia, hypoaesthesia, hypoglycemia, hypokalemia, hypotension, hypoxia, impaired concentration, impotence, increased LDH, involuntary muscle contractions, jaundice, leukocytosis, leukopenia, lymphadenopathy, malaise, manic reaction, mental deficiency, muscle weakness, myalgia, myocardial infarction, nervousness, palpitation, pancreatitis, paraesthesia, paralysis, paranoia, parosmia, phlebitis, pleural effusion, postural hypotension, pseudomembranous colitis, purpura, respiratory insufficiency, rhabdomyolysis, rigors, skin exfoliation, skin ulceration, sleep disorders, speech disorder, stupor, substernal chest pain, supraventricular tachycardia, syncope, synovitis, tachycardia, tendinitis, thrombocytopenia, tinnitus, tongue edema, tremor, urticaria, ventricular fibrillation, vertigo, weight decrease, WBC abnormal (not otherwise specified), withdrawal syndrome.
In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with other quinolones. The relationship of the drugs to these events is not presently established.
Crystalluria and cylindruria have been reported with other quinolones.
The following laboratory abnormalities appeared in 2.1 to 2.3% of patients receiving levofloxacin. It is not known whether these abnormalities were caused by the drug or the underlying condition being treated.
Blood Chemistry: decreased glucose
Hematology: decreased lymphocytes
Additional adverse events reported from worldwide post-marketing experience with levofloxacin include: allergic pneumonitis, anaphylactic shock, anaphylactoid reaction, dysphonia, abnormal EEG, encephalopathy, eosinophilia, erythema multiforme, hemolytic anemia, multi-system organ failure, increased International Normalized Ratio (INR)/prothrombin time, Stevens-Johnson Syndrome, tendon rupture, torsades de pointes, vasodilation.
Levofloxacin exhibits a low potential for acute toxicity. Mice, rats, dogs and monkeys exhibited the following clinical signs after receiving a single high dose of levofloxacin: ataxia, ptosis, decreased locomotor activity, dyspnea, prostration, tremors, and convulsions. Doses in excess of 1500 mg/kg orally and 250 mg/kg i.v. produced significant mortality in rodents. In the event of an acute overdosage, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
LEVAQUIN Injection should only be administered by intravenous infusion. It is not for intramuscular, intrathecal, intraperitoneal, or subcutaneous administration.
CAUTION: RAPID OR BOLUS INTRAVENOUS INFUSION MUST BE AVOIDED. Levofloxacin Injection should be infused intravenously slowly over a period of not less than 60 minutes. (See PRECAUTIONS .)
Single-use vials require dilution prior to administration. (See PREPARATION FOR ADMINISTRATION .)
The usual dose of LEVAQUIN Tablets/Injection is 500 mg administered orally or by slow infusion over 60 minutes every 24 hours as described in the following dosing chart. These recommendations apply to patients with normal renal function (i.e., creatinine clearance > 80 mL/min). For patients with altered renal function (i.e., creatinine clearance </= 80 mL/min), see the Patients with Impaired Renal Function subsection. Oral doses should be administered at least two hours before or two hours after antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or Videx®, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution.
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance.
Men: Creatinine Clearance (mL/min) =
|
Women: 0.85 × the value calculated for men.
The serum creatinine should represent a steady state of renal function.
LEVAQUIN Injection in Single-Use Vials: LEVAQUIN Injection is supplied in single-use vials containing a concentrated levofloxacin solution with the equivalent of 500 mg of levofloxacin in Water for Injection. The 20 mL vials contain 25 mg of levofloxacin/mL. THESE LEVAQUIN INJECTION SINGLE-USE VIALS MUST BE FURTHER DILUTED WITH AN APPROPRIATE SOLUTION PRIOR TO INTRAVENOUS ADMINISTRATION. (See COMPATIBLE INTRAVENOUS SOLUTIONS .) The concentration of the resulting diluted solution should be 5 mg/mL prior to administration.
This intravenous drug product should be inspected visually for particulate matter prior to administration. Samples containing visible particles should be discarded.
Since no preservative or bacteriostatic agent is present in this product, aseptic technique must be used in preparation of the final intravenous solution. Since the vials are for single-use only, any unused portion remaining in the vial should be discarded. When used to prepare two 250 mg doses, the full content of the vial should be withdrawn at once using a single-entry procedure, and a second dose should be prepared and stored for subsequent use. (See Stability of LEVAQUIN Injection Following Dilution .)
Since only limited data are available on the compatibility of levofloxacin intravenous injection with other intravenous substances, additives or other medications should not be added to LEVAQUIN Injection in single-use vials or infused simultaneously through the same intravenous line. If the same intravenous line is used for sequential infusion of several different drugs, the line should be flushed before and after infusion of LEVAQUIN Injection with an infusion solution compatible with LEVAQUIN Injection and with any other drug(s) administered via this common line.
Prepare the desired dosage of levofloxacin according to the following chart:
|
For example, to prepare a 500-mg dose using the 20 mL vial (25 mg/mL), withdraw 20 mL and dilute with a compatible intravenous solution to a total volume of 100 mL.
Compatible Intravenous Solutions: Any of the following intravenous solutions may be used to prepare a 5 mg/mL levofloxacin solution with the approximate pH values:
|
LEVAQUIN Injection Premix in Single-Use Flexible Containers: LEVAQUIN Injection is also supplied in 100 mL flexible containers containing a premixed, ready-to-use levofloxacin solution in D 5 W for single-use. The fill volume is either 50 or 100 mL. NO FURTHER DILUTION OF THIS PREPARATION IS NECESSARY. Consequently each 50 mL and 100 mL premix flexible container already contains a dilute solution with the equivalent of 250 mg and 500 mg of levofloxacin, respectively (5 mg/mL) in 5% Dextrose (D 5 W).
This parenteral drug product should be inspected visually for particulate matter prior to administration. Samples containing visible particles should be discarded.
Since the premix flexible containers are for single-use only, any unused portion should be discarded.
Since only limited data are available on the compatibility of levofloxacin intravenous injection with other intravenous substances, additives or other medications should not be added to LEVAQUIN Injection in flexible containers or infused simultaneously through the same intravenous line. If the same intravenous line is used for sequential infusion of several different drugs, the line should be flushed before and after infusion of LEVAQUIN Injection with an infusion solution compatible with LEVAQUIN Injection and with any other drug(s) administered via this common line.
Instructions for the Use of LEVAQUIN Injection Premix in Flexible Containers
To open:
Preparation for administration:
When stored under recommended conditions, LEVAQUIN Injection, as supplied in 20 mL vials and 100 mL flexible containers, is stable through the expiration date printed on the label.
LEVAQUIN Injection, when diluted in a compatible intravenous fluid to a concentration of 5 mg/mL, is stable for 72 h when stored at or below 25°C (77°F) and for 14 days when stored under refrigeration at 5°C (41°F) in plastic intravenous containers. Solutions that are diluted in a compatible intravenous solution and frozen in glass bottles or plastic intravenous containers are stable for 6 months when stored at -20°C (-4°F). THAW FROZEN SOLUTIONS AT ROOM TEMPERATURE 25°C (77°F) OR IN A REFRIGERATOR 8°C (46°F). DO NOT FORCE THAW BY MICROWAVE IRRADIATION OR WATER BATH IMMERSION. DO NOT REFREEZE AFTER INITIAL THAWING.
LEVAQUIN (levofloxacin tablets) Tablets are supplied as 250- and 500-mg modified rectangular, film-coated tablets.
LEVAQUIN Tablets are packaged in bottles and in unit-dose blister strips in the following configurations:
250-mg tablets: color: terra cotta pink
debossing: "LEVAQUIN" on side 1 and "250" on side 2
bottles of 50 (NDC 0045-1520-50)
unit-dose/100 tablets (NDC 0045-1520-10)
500-mg tablets: color: peach
debossing: "LEVAQUIN" on side 1 and "500" on side 2
bottles of 50 (NDC 0045-1525-50)
unit-dose/100 tablets (NDC 0045-1525-10)
LEVAQUIN Tablets should be stored at 15° to 30°C (59° to 86°F) in well-closed containers.
LEVAQUIN Tablets are manufactured for ORTHO-McNEIL PHARMACEUTICAL, INC. by Johnson & Johnson Pharmaceutical Partners, Gurabo, Puerto Rico 00778.
Single-Use Vials : LEVAQUIN (levofloxacin injection) Injection is supplied in single-use vials. Each vial contains a concentrated solution with the equivalent of 500 mg of levofloxacin.
25 mg/mL, 20 mL vials (NDC 0045-0069-51)
LEVAQUIN Injection in Single-Use Vials should be stored at controlled room temperature and protected from light.
LEVAQUIN Injection in Single-Use Vials is manufactured for ORTHO-McNEIL PHARMACEUTICAL, INC. by OMJ Pharmaceuticals, Inc., San German, Puerto Rico, 00683.
Premix in Flexible Containers : LEVAQUIN (levofloxacin injection) Injection is supplied as a single-use, premixed solution in flexible containers. Each bag contains a dilute solution with the equivalent of 250 mg or 500 mg of levofloxacin, respectively, in 5% Dextrose (D 5 W).
5 mg/mL (250 mg), 50 mL flexible container (NDC 0045-0067-01)
5 mg/mL (500 mg), 100 mL flexible container (NDC 0045-0068-01)
LEVAQUIN Injection Premix in Flexible Containers should be stored at or below 25°C (77°F); however, brief exposure up to 40°C (104°F) does not adversely affect the product. Avoid excessive heat and protect from freezing and light.
LEVAQUIN Injection Premix in Flexible Containers is manufactured for ORTHO-McNEIL PHARMACEUTICAL, INC. by ABBOTT Laboratories, North Chicago, IL 60064.
 |
 |
Adult inpatients and outpatients with a diagnosis of community-acquired bacterial pneumonia were evaluated in two pivotal clinical studies. In the first study, 590 patients were enrolled in a prospective, multi-center, unblinded randomized trial comparing levofloxacin 500 mg once daily orally or intravenously for 7 to 14 days to ceftriaxone 1 to 2 grams intravenously once or in equally divided doses twice daily followed by cefuroxime axetil 500 mg orally twice daily for a total of 7 to 14 days. Patients assigned to treatment with the control regimen were allowed to receive erythromycin (or doxycycline if intolerant of erythromycin) if an infection due to atypical pathogens was suspected or proven. Clinical and microbiologic evaluations were performed during treatment, 5 to 7 days posttherapy, and 3 to 4 weeks posttherapy. Clinical success (cure plus improvement) with levofloxacin at 5 to 7 days post-therapy, the primary efficacy variable in this study, was superior (95%) to the control group (83%) [95% CI of -19,-6]. In the second study, 264 patients were enrolled in a prospective, multi-center, non-comparative trial of 500 mg levofloxacin administered orally or intravenously once daily for 7 to 14 days. Clinical success for clinically evaluable patients was 93%. For both studies, the clinical success rate in patients with atypical pneumonia due to Chlamydia pneumoniae, Mycoplasma pneumoniae , and Legionella pneumophila were 96%, 96%, and 70%, respectively. Microbiologic eradication rates across both studies were as follows:
|
Additional studies were initiated to evaluate the utility of LEVAQUIN in community-acquired pneumonia due to S. pneumoniae , with particular interest in penicillin-resistant strains (MIC value for penicillin >/= 2 µg/mL). In addition to the studies previously discussed, inpatients and outpatients with mild to severe community-acquired pneumonia were evaluated in six additional clinical studies; one double-blind study, two open label randomized studies, and three open label non-comparative studies. The total number of clinically evaluable patients with S. pneumoniae across all 8 studies was 250 for levofloxacin and 41 for comparators. The clinical success rate (cured or improved) among the 250 levofloxacin-treated patients with S. pneumoniae was 245/250 (98%). The clinical success rate among the 41 comparator-treated patients with S. pneumoniae was 39/41 (95%).
Across these 8 studies, 18 levofloxacin-treated and 4 non-quinolone comparator-treated patients with community-acquired pneumonia due to penicillin-resistant S. pneumoniae (MIC value for penicillin >/= 2 µg/mL) were identified. Of the 18 levofloxacin-treated patients, 15 were evaluable following the completion of therapy. Fifteen out of the 15 evaluable levofloxacin-treated patients with community-acquired pneumonia due to penicillin-resistant S. pneumoniae achieved clinical success (cure or improvement). Of these 15 patients, 6 were bacteremic and 5 were classified as having severe disease. Of the 4 comparator-treated patients with community-acquired pneumonia due to penicillin-resistant S. pneumoniae , 3 were evaluable for clinical efficacy. Three out of the 3 evaluable comparator-treated patients achieved clinical success. All three of the comparator-treated patients were bacteremic and had disease classified as severe.
Levofloxacin and other quinolones have been shown to cause arthropathy in immature animals of most species tested. (See .) In immature dogs (4-5 months old), oral doses of 10 mg/kg/day for 7 days and intravenous doses of 4 mg/kg/day for 14 days of levofloxacin resulted in arthropathic lesions. Administration at oral doses of 300 mg/kg/day for 7 days and intravenous doses of 60 mg/kg/day for 4 weeks produced arthropathy in juvenile rats.
When tested in a mouse ear swelling bioassay, levofloxacin exhibited phototoxicity similar in magnitude to ofloxacin, but less phototoxicity than other quinolones.
While crystalluria has been observed in some intravenous rat studies, urinary crystals are not formed in the bladder, being present only after micturition and are not associated with nephrotoxicity.
In mice, the CNS stimulatory effect of quinolones is enhanced by concomitant administration of nonsteroidal anti-inflammatory drugs.
In dogs, levofloxacin administered at 6 mg/kg or higher by rapid intravenous injection produced hypotensive effects. These effects were considered to be related to histamine release.
In vitro and in vivo studies in animals indicate that levofloxacin is neither an enzyme inducer or inhibitor in the human therapeutic plasma concentration range; therefore, no drug metabolizing enzyme-related interactions with other drugs or agents are anticipated.
LEVAQUIN®
(levofloxacin tablets)
250 mg Tablets and 500 mg Tablets
This leaflet contains important information about LEVAQUIN® (levofloxacin), and should be read completely before you begin treatment. This leaflet does not take the place of discussions with your doctor or health care professional about your medical condition or your treatment. This leaflet does not list all benefits and risks of LEVAQUIN®. The medicine described here can be prescribed only by a licensed health care professional. If you have any questions about LEVAQUIN® talk to your health care professional. Only your health care professional can determine if LEVAQUIN® is right for you.
LEVAQUIN® is a quinolone antibiotic used to treat lung, sinus, skin, and urinary tract infections caused by certain germs called bacteria. LEVAQUIN® kills many of the types of bacteria that can infect the lungs, sinuses, skin, and urinary tract and has been shown in a large number of clinical trials to be safe and effective for the treatment of bacterial infections.
Sometimes viruses rather than bacteria may infect the lungs and sinuses (for example the common cold). LEVAQUIN®, like other antibiotics, does not kill viruses.
You should contact your health care professional if you think that your condition is not improving while taking LEVAQUIN®. LEVAQUIN® Tablets are either terra cotta pink for the 250 mg tablet or peach colored for the 500 mg tablet.
LEVAQUIN® should be taken once a day for 3, 7, 10, or 14 days depending on your prescription. It should be swallowed and may be taken with or without food. Try to take the tablet at the same time each day and drink fluids liberally.
You may begin to feel better quickly; however, in order to make sure that all bacteria are killed, you should complete the full course of medication. Do not take more than the prescribed dose of LEVAQUIN® even if you missed a dose by mistake. You should not take a double dose.
You should not take LEVAQUIN® if you have ever had a severe allergic reaction to any of the group of antibiotics known as "quinolones" such as ciprofloxacin. Serious and occasionally fatal allergic reactions have been reported in patients receiving therapy with quinolones, including LEVAQUIN®.
If you are pregnant or are planning to become pregnant while taking LEVAQUIN®, talk to your health care professional before taking this medication. LEVAQUIN® is not recommended for use during pregnancy or nursing, as the effects on the unborn child or nursing infant are unknown.
LEVAQUIN® is not recommended for children.
LEVAQUIN® is generally well tolerated. The most common side effects caused by LEVAQUIN®, which are usually mild, include nausea, diarrhea, itching, abdominal pain, dizziness, flatulence, rash and vaginitis in women.
You should be careful about driving or operating machinery until you are sure LEVAQUIN® is not causing dizziness.
Allergic reactions have been reported in patients receiving quinolones including LEVAQUIN®, even after just one dose. If you develop hives, skin rash or other symptoms of an allergic reaction, you should stop taking this medication and call your health care professional.
Ruptures of shoulder, hand, or Achilles tendons have been reported in patients receiving quinolones, including LEVAQUIN®. If you develop pain, swelling, or rupture of a tendon you should stop taking LEVAQUIN® and contact your health care professional.
Some quinolone antibiotics have been associated with the development of phototoxicity ("sunburns" and "blistering sunburns") following exposure to sunlight or other sources of ultraviolet light such as artificial ultraviolet light used in tanning salons. LEVAQUIN® has been infrequently associated with phototoxicity. You should avoid excessive exposure to sunlight or artificial ultraviolet light while you are taking LEVAQUIN®.
If you have diabetes and you develop a hypoglycemic reaction while on LEVAQUIN®, you should stop taking LEVAQUIN® and call your health care professional.
Convulsions have been reported in patients receiving quinolone antibiotics including LEVAQUIN®. If you have experienced convulsions in the past, be sure to let your physician know that you have a history of convulsions.
Quinolones, including LEVAQUIN®, may also cause central nervous system stimulation which may lead to tremors, restlessness, anxiety, lightheadedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and, rarely, suicidal thoughts or acts.
If you notice any side-effects not mentioned in this leaflet or you have concerns about the side effects you are experiencing, please inform your health care professional.
For more complete information regarding levofloxacin, please refer to the full prescribing information, which may be obtained from your health care professional, pharmacist, or the Physicians Desk Reference (PDR).
Taking warfarin (Coumadin®) and LEVAQUIN® together can further predispose you to the development of bleeding problems. If you take warfarin, be sure to tell your health care professional.
Many antacids and multivitamins may interfere with the absorption of LEVAQUIN® and may prevent it from working properly. You should take LEVAQUIN® either 2 hours before or 2 hours after taking these products.
It is important to let your health care professional know all of the medicines you are using.
Take your dose of LEVAQUIN® once a day.
Complete the course of medication even if you are feeling better.
Keep this medication out of the reach of children.
This information does not take the place of discussions with your doctor or health care professional about your medical condition on your treatment.
ORTHO-McNEIL
OMP DIVISION
ORTHO-McNEIL PHARMACEUTICAL, INC.
Raritan, New Jersey 08869
U.S. Patent No. 4,382,892 and U.S. Patent No. 5,053,407
© OMP 2000 Revised February 2000 7517800