 |
|
|
OxyContin® (oxycodone hydrochloride controlled-release) tablets are an opioid analgesic supplied in 10 mg, 20 mg, 40 mg, 80 mg, and 160 mg tablet strengths for oral administration. The tablet strengths describe the amount of oxycodone per tablet as the hydrochloride salt. The structural formula for oxycodone hydrochloride is as follows:
 |
The chemical formula is 4, 5-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride.
Oxycodone is a white, odorless crystalline powder derived from the opium alkaloid, thebaine. Oxycodone hydrochloride dissolves in water (1 g in 6 to 7 mL). It is slightly soluble in alcohol (octanol water partition coefficient 0.7). The tablets contain the following inactive ingredients: ammonio methacrylate copolymer, hydroxypropyl methylcellulose, lactose, magnesium stearate, povidone, red iron oxide (20 mg strength tablet only), stearyl alcohol, talc, titanium dioxide, triacetin, yellow iron oxide (40 mg strength tablet only), yellow iron oxide with FD&C blue No. 2 (80 mg strength tablet only), FD&C blue No. 2 (160 mg strength tablet only) and other ingredients.
OxyContin® 80 mg and 160 mg Tablets ARE FOR USE IN OPIOID TOLERANT PATIENTS ONLY.
Oxycodone is a pure agonist opioid whose principal therapeutic action is analgesia. Other therapeutic effects of oxycodone include anxiolysis, euphoria and feelings of relaxation. Like all pure opioid agonists, there is no ceiling effect to analgesia, such as is seen with partial agonists or non-opioid analgesics.
The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and play a role in the analgesic effects of this drug.
Oxycodone produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves both a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
Oxycodone depresses the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia.
Oxycodone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic. Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Gastrointestinal Tract and Other Smooth Muscle
Oxycodone causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in gastric, biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Oxycodone may produce release of histamine with or without associated peripheral vasodilation. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
Concentration--Efficacy Relationships (Pharmacodynamics)
Studies in normal volunteers and patients reveal predictable relationships between oxycodone dosage and plasma oxycodone concentrations, as well as between concentration and certain expected opioid effects. In normal volunteers these include pupillary constriction, sedation and overall "drug effect" and in patients, analgesia and feelings of "relaxation." In non-tolerant patients, analgesia is not usually seen at a plasma oxycodone concentration of less than 5-10 ng/mL.
As with all opioids, the minimum effective plasma concentration for analgesia will vary widely among patients, especially among patients who have been previously treated with potent agonist opioids. As a result, patients need to be treated with individualized titration of dosage to the desired effect. The minimum effective analgesic concentration of oxycodone for any individual patient may increase with repeated dosing due to an increase in pain and/or the development of tolerance.
Concentration--Adverse Experience Relationships
OxyContin tablets are associated with typical opioid-related adverse experiences similar to those seen with immediate-release oxycodone and all opioids. There is a general relationship between increasing oxycodone plasma concentration and increasing frequency of dose-related opioid adverse experiences such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation is altered by the development of tolerance to opioid-related side effects, and the relationship is poorly understood.
As with all opioids, the dose must be individualized (see DOSAGE AND ADMINISTRATION ), because the effective analgesic dose for some patients will be too high to be tolerated by other patients.
The activity of OxyContin® (oxycodone hydrochloride controlled-release) tablets is primarily due to the parent drug oxycodone. OxyContin tablets are designed to provide controlled delivery of oxycodone over 12 hours. Oxycodone release from OxyContin tablets is pH independent. Oxycodone is well absorbed from OxyContin tablets with an oral bioavailability of from 60% to 87%. The relative oral bioavailability of OxyContin to immediate-release oral dosage forms is 100%. Upon repeated dosing in normal volunteers, steady-state levels were achieved within 24-36 hours. Dose proportionality and/or bioavailability has been established for the 10 mg, 20 mg, 40 mg, 80 mg, and 160 mg tablet strengths for both peak plasma levels (C max ) and extent of absorption (AUC). Oxycodone is extensively metabolized and eliminated primarily in the urine as both conjugated and unconjugated metabolites. The apparent elimination half-life of oxycodone following the administration of OxyContin was 4.5 hours compared to 3.2 hours for immediate-release oxycodone.
Absorption
About 60% to 87% of an oral dose of oxycodone reaches the central compartment in comparison to a parenteral dose. This high oral bioavailability is due to low pre-systemic and/or first-pass metabolism. In normal volunteers the t ½ of absorption is 0.4 hours for immediate-release oral oxycodone. In contrast, OxyContin tablets exhibit a biphasic absorption pattern with two apparent absorption half-times of 0.6 and 6.9 hours, which describes the initial release of oxycodone from the tablet followed by a prolonged release.
Dose proportionality has been established for the 10 mg, 20 mg, 40 mg, and 80 mg tablet strengths for both peak plasma concentrations (C max ) and extent of absorption (AUC) (see Table 1 below). Another study established that the 160 mg tablet is bioequivalent to 2 × 80 mg tablets as well as to 4 × 40 mg for both peak plasma concentrations (C max ) and extent of absorption (AUC) (see Table 2 below). Given the short half-life of elimination of oxycodone from OxyContin, steady-state plasma concentrations of oxycodone are
 |
|
|
||||||||||||||||||||||||||||||
Food Effects
Food has no significant effect on the extent of absorption of oxycodone from OxyContin. However, the peak plasma concentration of oxycodone increased by 25% when OxyContin 160 mg tablet was administered with a high fat meal.
Distribution
Following intravenous administration, the volume of distribution (Vss) for oxycodone was 2.6 L/kg. Oxycodone binding to plasma protein at 37°C and a pH of 7.4 was about 45%. Once absorbed, oxycodone is distributed to skeletal muscle, liver, intestinal tract, lungs, spleen, and brain. Oxycodone has been found in breast milk (see PRECAUTIONS ).
Oxycodone hydrochloride is extensively metabolized to noroxycodone, oxymorphone, and their glucuronides. The major circulating metabolite is noroxycodone with an AUC ratio of 0.6 relative to that of oxycodone. Noroxycodone is reported to be a considerably weaker analgesic than oxycodone. Oxymorphone, although possessing analgesic activity, is present in the plasma only in low concentrations. The correlation between oxymorphone concentrations and opioid effects was much less than that seen with oxycodone plasma concentrations. The analgesic activity profile of other metabolites is not known at present.
The formation of oxymorphone, but not noroxycodone, is mediated by CYP2D6 and as such its formation can, in theory, be affected by other drugs (see Drug-Drug Interactions ).
Oxycodone and its metabolites are excreted primarily via the kidney. The amounts measured in the urine have been reported as follows: free oxycodone up to 19%; conjugated oxycodone up to 50%; free oxymorphone 0%; conjugated oxymorphone </=14%; both free and conjugated noroxycodone have been found in the urine but not quantified. The total plasma clearance was 0.8 L/min for adults.
Special Populations
Elderly
The plasma concentrations of oxycodone are only nominally affected by age, being 15% greater in elderly as compared to young subjects. There were no differences in adverse event reporting between young and elderly subjects.
Female subjects have, on average, plasma oxycodone concentrations up to 25% higher than males on a body weight adjusted basis. The reason for this difference is unknown.
Preliminary data from a study involving patients with mild to severe renal dysfunction (creatinine clearance <60 mL/min) show peak plasma oxycodone and noroxycodone concentrations 50% and 20% higher, respectively and AUC values for oxycodone, noroxycodone, and oxymorphone 60%, 50%, and 40% higher than normal subjects, respectively. This is accompanied by an increase in sedation but not by differences in respiratory rate, pupillary constriction, or several other measures of drug effect. There was an increase in t ½ of elimination for oxycodone of only 1 hour (see PRECAUTIONS ).
Preliminary data from a study involving patients with mild to moderate hepatic dysfunction show peak plasma oxycodone and noroxycodone concentrations 50% and 20% higher, respectively, than normal subjects. AUC values are 95% and 65% higher, respectively. Oxymorphone peak plasma concentrations and AUC values are lower by 30% and 40%. These differences are accompanied by increases in some, but not other, drug effects. The t ½ elimination for oxycodone increased by 2.3 hours (see PRECAUTIONS ).
Rectal Administration
Rectal administration of OxyContin tablets is not recommended. Preliminary data from a study involving 21 normal volunteers, show OxyContin tablets administered per rectum resulted in an AUC 39% greater and a C max 9% higher than tablets administered by mouth (see PRECAUTIONS ).
Drug-Drug Interactions (see PRECAUTIONS )
Oxycodone is metabolized in part via CYP2D6 to oxymorphone which represents less than 15% of the total administered dose. This route of elimination can be blocked by a variety of drugs (e.g., certain cardiovascular drugs and anti-depressants). Patients receiving such drugs concomitantly with OxyContin do not appear to present different therapeutic profiles than other patients.
OxyContin® (oxycodone hydrochloride controlled-release) tablets were evaluated in studies involving 713 patients with either cancer or non-cancer pain. All patients receiving OxyContin were dosed q12h. Efficacy comparable to other forms of oral oxycodone was demonstrated in clinical studies using pharmacokinetic, pharmacodynamic, and efficacy outcomes. The outcome of these trials indicated: (1) a positive relationship between dose and plasma oxycodone concentration, (2) a positive relationship between plasma oxycodone concentration and analgesia, and (3) an observed peak to trough variation in plasma concentration with OxyContin lying within the observed range established with qid dosing of immediate-release oxycodone in clinical populations at the same total daily dose.
In clinical trials, OxyContin tablets were substituted for a wide variety of analgesics, including acetaminophen (APAP), aspirin (ASA), other non-steroidal anti-inflammatory drugs (NSAIDs), opioid combination products and single-entity opioids, primarily morphine. In cancer patients receiving adequate opioid therapy at baseline, pain intensity scores and acceptability of therapy remained unchanged by transfer to OxyContin. For non-cancer pain patients who had moderate to severe pain at baseline on prn opioid therapy, pain control and acceptability of therapy improved with the introduction of fixed-interval therapy with OxyContin.
OxyContin was studied in three double-blind, controlled clinical trials involving 341 cancer patients and several open-label trials with therapy durations of over 10 months.
Two, double-blind, controlled clinical studies indicated that OxyContin dosed q12h produced analgesic efficacy equivalent to immediate-release oxycodone dosed qid at the same total daily dose. Peak and trough plasma concentrations attained were similar to those attained with immediate-release oxycodone at equivalent total daily doses. With titration to analgesic effect and proper use of rescue medication, nearly every patient achieved adequate pain control with OxyContin.
In the third study, a double-blind, active-controlled, crossover trial, OxyContin dosed q12h was shown to be equivalent in efficacy and safety to immediate-release oxycodone dosed qid at the same total daily dose. Patients were able to be titrated to an acceptable analgesic effect with either OxyContin or immediate-release oxycodone with both treatments providing stable pain control within 2 days in most patients.
In patients with cancer pain, the total daily OxyContin doses tested ranged from 20 mg to 640 mg per day. The average total daily dose was approximately 105 mg per day.
A double-blind, placebo-controlled, fixed-dose, parallel group study was conducted in 133 patients with moderate to severe osteoarthritis pain, who were judged as having inadequate pain control with prn opioids and maximal non-steroidal anti-inflammatory therapy. In this study, 20 mg OxyContin q12h significantly decreased pain and improved quality of life, mood, and sleep, relative to placebo. Both dose-concentration and concentration-effect relationships were noted with a minimum effective plasma oxycodone concentration of approximately 5-10 ng/mL.
In a double-blind, active-controlled, crossover study involving 57 patients with low-back pain inadequately controlled with prn opioids and non-opioid therapy, OxyContin administered q12h provided analgesia equivalent to immediate-release oxycodone administered qid. Patients could be titrated to an acceptable analgesic effect with either OxyContin or immediate-release forms of oxycodone.
Single-Dose Comparison with Standard Therapy
A single-dose, double-blind, placebo-controlled, post-operative study of 182 patients was conducted utilizing graded doses of OxyContin (10, 20, and 30 mg). Twenty and 30 mg of OxyContin gave equivalent peak analgesic effect compared to two oxycodone 5 mg/acetaminophen 325 mg tablets and to 15 mg immediate-release oxycodone, while the 10 mg dose of OxyContin was intermediate between both the immediate-release and combination products and placebo. The onset of analgesic action with OxyContin occurred within 1 hour in most patients following oral administration.
OxyContin is not recommended pre-operatively (preemptive analgesia) or for the management of pain in the immediate post-operative period (the first 12 to 24 hours following surgery) because the safety or appropriateness of fixed-dose, long-acting opioids in this setting has not been established.
Other Clinical Trials
In open-label trials involving approximately 200 patients with cancer-related and non-cancer pain, dosed according to the package insert recommendations, appropriate analgesic effectiveness was noted without regard to age, gender, race, or disease state. There were no unusual drug interactions observed in patients receiving a wide range of medications common in these populations.
For opioid-naive patients, the average total daily dose of OxyContin was approximately 40 mg per day. There was no evidence of oxycodone and metabolite accumulation during 8 months of therapy. For cancer pain patients the average total daily dose was 105 mg (range 20 to 720 mg) per day. There was a significant decrease in acute opioid-related side effects, except for constipation, during the first several weeks of therapy. Development of significant tolerance to analgesia was uncommon.
A cohort of patients have been treated with OxyContin 80 mg tablets. There were no differences in the efficacy or safety profiles than seen with the other tablet strengths.
OxyContin® tablets are a controlled-release oral formulation of oxycodone hydrochloride indicated for the management of moderate to severe pain where use of an opioid analgesic is appropriate for more than a few days (see CLINICAL PHARMACOLOGY ; CLINICAL TRIALS ).
OxyContin® is contraindicated in patients with known hypersensitivity to oxycodone, or in any situation where opioids are contraindicated. This includes patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment), and patients with acute or severe bronchial asthma or hypercarbia. OxyContin is contraindicated in any patient who has or is suspected of having paralytic ileus.
OxyContin® (oxycodone hydrochloride controlled-release) TABLETS ARE TO BE SWALLOWED WHOLE, AND ARE NOT TO BE BROKEN, CHEWED OR CRUSHED. TAKING BROKEN, CHEWED OR CRUSHED OxyContin TABLETS COULD LEAD TO THE RAPID RELEASE AND ABSORPTION OF A POTENTIALLY TOXIC DOSE OF OXYCODONE.
Respiratory depression is the chief hazard from all opioid agonist preparations. Respiratory depression occurs most frequently in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration.
Oxycodone should be used with extreme caution in patients with significant chronic obstructive pulmonary disease or cor pulmonale, and in patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or preexisting respiratory depression. In such patients, even usual therapeutic doses of oxycodone may decrease respiratory drive to the point of apnea. In these patients alternative non-opioid analgesics should be considered, and opioids should be employed only under careful medical supervision at the lowest effective dose.
Head Injury
The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, and may be markedly exaggerated in the presence of head injury, intracranial lesions, or other sources of preexisting increased intracranial pressure. Oxycodone produces effects on pupillary response and consciousness which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries.
OxyContin®, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents which compromise vasomotor tone. OxyContin may produce orthostatic hypotension in ambulatory patients. OxyContin, like all opioid analgesics, should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
OxyContin® 80 mg and 160 mg Tablets are for use only in opioid tolerant patients requiring daily oxycodone equivalent dosages of 160 mg or more for the 80 mg tablet and 320 mg or more for the 160 mg tablet. Care should be taken in the prescription of this tablet strength. Patients should be instructed against use by individuals other than the patient for whom it was prescribed, as such inappropriate use may have severe medical consequences.
One OxyContin® 160 mg tablet is comparable to two 80 mg tablets when taken on an empty stomach. With a high fat meal, however, there is a 25% greater peak plasma concentration following one 160 mg tablet. Dietary caution should be taken when patients are initially titrated to 160 mg tablets (see DOSAGE AND ADMINISTRATION ).
General
OxyContin® (oxycodone hydrochloride controlled-release) tablets are intended for use in patients who require oral pain therapy with an opioid agonist of more than a few days duration. As with any opioid analgesic, it is critical to adjust the dosing regimen individually for each patient (see DOSAGE AND ADMINISTRATION ).
Selection of patients for treatment with OxyContin should be governed by the same principles that apply to the use of similar controlled-release opioid analgesics (see ). Opioid analgesics given on a fixed-dosage schedule have a narrow therapeutic index in certain patient populations, especially when combined with other drugs, and should be reserved for cases where the benefits of opioid analgesia outweigh the known risks of respiratory depression, altered mental state, and postural hypotension. Physicians should individualize treatment in every case, using non-opioid analgesics, prn opioids and/or combination products, and chronic opioid therapy with drugs such as OxyContin in a progressive plan of pain management such as outlined by the World Health Organization, the Agency for Health Care Policy and Research, and the American Pain Society.
Use of OxyContin is associated with increased potential risks and should be used only with caution in the following conditions: acute alcoholism; adrenocortical insufficiency (e.g., Addison' disease); CNS depression or coma; delirium tremens; debilitated patients; kyphoscoliosis associated with respiratory depression; myxedema or hypothyroidism; prostatic hypertrophy or urethral stricture; severe impairment of hepatic, pulmonary or renal function; and toxic psychosis.
The administration of oxycodone, like all opioid analgesics, may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Oxycodone may aggravate convulsions in patients with convulsive disorders, and all opioids may induce or aggravate seizures in some clinical settings.
Interactions with other CNS Depressants
OxyContin, like all opioid analgesics, should be used with caution and started in a reduced dosage ( 1 / 3 to 1 / 2 of the usual dosage) in patients who are concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, other tranquilizers, and alcohol. Interactive effects resulting in respiratory depression, hypotension, profound sedation, or coma may result if these drugs are taken in combination with the usual doses of OxyContin.
Interactions with Mixed Agonist/Antagonist Opioid Analgesics
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol, and buprenorphine) should be administered with caution to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as oxycodone. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of oxycodone and/or may precipitate withdrawal symptoms in these patients.
OxyContin is not recommended pre-operatively (preemptive analgesia) or for the management of pain in the immediate post-operative period (the first 12 to 24 hours following surgery) for patients not previously taking the drug, because its safety in this setting has not been established.
Patients who are already receiving OxyContin tablets as part of ongoing analgesic therapy may be safely continued on the drug if appropriate dosage adjustments are made considering the procedure, other drugs given and the temporary changes in physiology caused by the surgical intervention (see PRECAUTIONS : Drug-Drug Interactions , and DOSAGE AND ADMINISTRATION ).
Morphine and other opioids have been shown to decrease bowel motility. Ileus is a common post-operative complication, especially after intra-abdominal surgery with opioid analgesia. Caution should be taken to monitor for decreased bowel motility in post-operative patients receiving opioids. Standard supportive therapy should be implemented.
Use in Pancreatic/Biliary Tract Disease
Oxycodone may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids like oxycodone may cause increases in the serum amylase level.
Tolerance and Physical Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is the occurrence of withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
Significant tolerance should not occur in most of the patients treated with the lowest doses of oxycodone. It should be expected, however, that a fraction of cancer patients will develop some degree of tolerance and require progressively higher dosages of OxyContin to maintain pain control during chronic treatment. Regardless of whether this occurs as a result of increased pain secondary to disease progression or pharmacological tolerance, dosages can usually be increased safely by adjusting the patient' dose to maintain an acceptable balance between pain relief and side effects. The dosage should be selected according to the patient' individual analgesic response and ability to tolerate side effects. Tolerance to the analgesic effect of opioids is usually paralleled by tolerance to side effects, except for constipation.
Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug or may be precipitated through the administration of drugs with opioid antagonist activity (see OVERDOSAGE ). If OxyContin is abruptly discontinued in a physically dependent patient, an abstinence syndrome may occur. This is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
If signs and symptoms of withdrawal occur, patients should be treated by reinstitution of opioid therapy followed by a gradual, tapered dose reduction of OxyContin combined with symptomatic support (see DOSAGE AND ADMINISTRATION : Cessation of Therapy ).
Information for Patients/Caregivers
If clinically advisable, patients receiving OxyContin (oxycodone hydrochloride controlled-release) tablets or their caregivers should be given the following information by the physician, nurse, pharmacist, or caregiver:
Due to the broad range of plasma concentrations seen in clinical populations, the varying degrees of pain, and the development of tolerance, plasma oxycodone measurements are usually not helpful in clinical management. Plasma concentrations of the active drug substance may be of value in selected, unusual or complex cases.
Interactions with Alcohol and Drugs of Abuse
Oxycodone may be expected to have additive effects when used in conjunction with alcohol, other opioids or illicit drugs which cause central nervous system depression.
Use in Drug and Alcohol Addiction
OxyContin is an opioid with no approved use in the management of addictive disorders. Its proper usage in individuals with drug or alcohol dependence, either active or in remission, is for the management of pain requiring opioid analgesia.
Opioid analgesics, including OxyContin, may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
Oxycodone is metabolized in part to oxymorphone via CYP2D6. While this pathway may be blocked by a variety of drugs (e.g., certain cardiovascular drugs and antidepressants), such blockade has not yet been shown to be of clinical significance with this agent. Clinicians should be aware of this possible interaction, however.
Use with CNS Depressants
OxyContin, like all opioid analgesics, should be started at 1 / 3 to 1 / 2 of the usual dosage in patients who are concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, centrally acting anti-emetics, tranquilizers, and alcohol because respiratory depression, hypotension, and profound sedation or coma may result. No specific interaction between oxycodone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate.
Mutagenicity/Carcinogenicity
Oxycodone was not mutagenic in the following assays: Ames Salmonella and E. Coli test with and without metabolic activation at doses of up to 5000 µg, chromosomal aberration test in human lymphocytes in the absence of metabolic activation at doses of up to 1500 µg/ml and with activation 48 hours after exposure at doses of up to 5000 µg/ml, and in the in vivo bone marrow micronucleus test in mice (at plasma levels of up to 48 µg/ml). Mutagenic results occurred in the presence of metabolic activation in the human chromosomal aberration test (at greater than or equal to 1250 µg/ml) at 24 but not 48 hours of exposure and in the mouse lymphoma assay at doses of 50 µg/ml or greater with metabolic activation and at 400 µg/ml or greater without metabolic activation. The data from these tests indicate that the genotoxic risk to humans may be considered low.
Studies of oxycodone in animals to evaluate its carcinogenic potential have not been conducted owing to the length of clinical experience with the drug substance.
Pregnancy
Teratogenic Effects--Category B: Reproduction studies have been performed in rats and rabbits by oral administration at doses up to 8 mg/kg (48 mg/m 2 ) and 125 mg/kg (1375 mg/m 2 ), respectively. These doses are 3 and 47 times a human dose of 160 mg/day (90 mg/m 2 ), based on mg/kg of a 60 kg adult (0.5 and 15 times this human dose based upon mg/m 2 ). The results did not reveal evidence of harm to the fetus due to oxycodone. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects--Neonates whose mothers have been taking oxycodone chronically may exhibit respiratory depression and/or withdrawal symptoms, either at birth and/or in the nursery.
OxyContin is not recommended for use in women during and immediately prior to labor and delivery because oral opioids may cause respiratory depression in the newborn.
Nursing Mothers
Low concentrations of oxycodone have been detected in breast milk. Withdrawal symptoms can occur in breast-feeding infants when maternal administration of an opioid analgesic is stopped. Ordinarily, nursing should not be undertaken while a patient is receiving OxyContin since oxycodone may be excreted in the milk.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 have not been established with this dosage form of oxycodone. However, oxycodone has been used extensively in the pediatric population in other dosage forms, as have the excipients used in this formulation. No specific increased risk is expected from the use of this form of oxycodone in pediatric patients old enough to safely take tablets if dosing is adjusted for the patient's weight (see DOSAGE AND ADMINISTRATION ). It must be remembered that OxyContin tablets cannot be crushed or divided for administration.
Geriatric Use
In controlled pharmacokinetic studies in elderly subjects (greater than 65 years) the clearance of oxycodone appeared to be slightly reduced. Compared to young adults, the plasma concentrations of oxycodone were increased approximately 15%. In clinical trials with appropriate initiation of therapy and dose titration, no untoward or unexpected side effects were seen based on age, and the usual doses and dosing intervals are appropriate for the geriatric patient. As with all opioids, the starting dose should be reduced to 1 / 3 to 1 / 2 of the usual dosage in debilitated, non-tolerant patients.
A study of OxyContin in patients with hepatic impairment indicates greater plasma concentrations than those with normal function. The initiation of therapy at 1 / 3 to 1 / 2 the usual doses and careful dose titration is warranted.
In patients with renal impairment, as evidenced by decreased creatinine clearance (<60 mL/min.), the concentrations of oxycodone in the plasma are approximately 50% higher than in subjects with normal renal function. Dose initiation should follow a conservative approach. Dosages should be adjusted according to the clinical situation.
Gender Differences
In pharmacokinetic studies, opioid-naive females demonstrate up to 25% higher average plasma concentrations and greater frequency of typical opioid adverse events than males, even after adjustment for body weight. The clinical relevance of a difference of this magnitude is low for a drug intended for chronic usage at individualized dosages, and there was no male/female difference detected for efficacy or adverse events in clinical trials.
Rectal Administration
OxyContin® Tablets are not recommended for administration per rectum. A study in normal volunteers showed a significantly greater AUC and higher C max during this route of administration (see PHARMACOKINETICS AND METABOLISM ).
Serious adverse reactions which may be associated with OxyContin® (oxycodone hydrochloride controlled-release) tablet therapy in clinical use are those observed with other opioid analgesics, including: respiratory depression, apnea, respiratory arrest, and (to an even lesser degree) circulatory depression, hypotension, or shock (see OVERDOSAGE ). The non-serious adverse events seen on initiation of therapy with OxyContin are typical opioid side effects. These events are dose-dependent, and their frequency depends upon the dose, the clinical setting, the patient' level of opioid tolerance, and host factors specific to the individual. They should be expected and managed as a part of opioid analgesia. The most frequent (>5%) include constipation, nausea, somnolence, dizziness, vomiting, pruritus, headache, dry mouth, sweating, and asthenia.
In many cases the frequency of these events during initiation of therapy may be minimized by careful individualization of starting dosage, slow titration, and the avoidance of large swings in the plasma concentrations of the opioid. Many of these adverse events will cease or decrease in intensity as OxyContin therapy is continued and some degree of tolerance is developed.
In clinical trials comparing OxyContin with immediate-release oxycodone and placebo, the most common adverse events (>5%) reported by patients (pts) at least once during therapy were:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The following adverse experiences were reported in OxyContin treated patients with an incidence between 1% and 5%. In descending order of frequency they were anorexia, nervousness, insomnia, fever, confusion, diarrhea, abdominal pain, dyspepsia, rash, anxiety, euphoria, dyspnea, postural hypotension, chills, twitching, gastritis, abnormal dreams, thought abnormalities, and hiccups.
The following adverse reactions occurred in less than 1% of patients involved in clinical trials:
General: accidental injury, chest pain, facial edema, malaise, neck pain, pain
Cardiovascular: migraine, syncope, vasodilation, ST depression
Digestive: dysphagia, eructation, flatulence, gastrointestinal disorder, increased appetite, nausea and vomiting, stomatitis, ileus
Hemic and Lymphatic: lymphadenopathy
Metabolic and Nutritional: dehydration, edema, hyponatremia, peripheral edema, syndrome of inappropriate anti-diuretic hormone secretion, thirst
Nervous: abnormal gait, agitation, amnesia, depersonalization, depression, emotional lability, hallucination, hyperkinesia, hypesthesia, hypotonia, malaise, paresthesia, seizures, speech disorder, stupor, tinnitus, tremor, vertigo, withdrawal syndrome with or without seizures
Respiratory cough increased, pharyngitis, voice alteration
Skin: dry skin, exfoliative dermatitis, urticaria
Special Senses: abnormal vision, taste perversion
Urogenital: dysuria, hematuria, impotence, polyuria, urinary retention, urination impaired
OxyContin® is a mu-agonist opioid with an abuse liability similar to morphine and is a Schedule II controlled substance. Oxycodone products are common targets for both drug abusers and drug addicts. Delayed absorption, as provided by OxyContin tablets, is believed to reduce the abuse liability of a drug.
Drug addiction (drug dependence, psychological dependence) is characterized by a preoccupation with the procurement, hoarding, and abuse of drugs for non-medicinal purposes. Drug dependence is treatable, utilizing a multi-disciplinary approach, but relapse is common. Iatrogenic "addiction" to opioids legitimately used in the management of pain is very rare. "Drug seeking" behavior is very common to addicts. Tolerance and physical dependence in pain patients are not signs of psychological dependence. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control. Most chronic pain patients limit their intake of opioids to achieve a balance between the benefits of the drug and dose-limiting side effects.
Physicians should be aware that psychological dependence may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true psychological dependence and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances.
OxyContin consists of a dual-polymer matrix, intended for oral use only. Parenteral venous injection of the tablet constituents, especially talc, can be expected to result in local tissue necrosis and pulmonary granulomas.
Acute overdosage with oxycodone can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension, and death.
In the treatment of oxycodone overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The pure opioid antagonists such as naloxone or nalmefene are specific antidotes against respiratory depression from opioid overdose. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to oxycodone overdose. They should be administered cautiously to persons who are known, or suspected to be, physically dependent on any opioid agonist including OxyContin®. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Please see the prescribing information for the specific opioid antagonist for details of their proper use.
General Principles
OxyContin® (oxycodone hydrochloride controlled-release) TABLETS ARE TO BE SWALLOWED WHOLE, AND ARE NOT TO BE BROKEN, CHEWED OR CRUSHED. TAKING BROKEN, CHEWED OR CRUSHED OxyContin TABLETS COULD LEAD TO THE RAPID RELEASE AND ABSORPTION OF A POTENTIALLY TOXIC DOSE OF OXYCODONE.
ONE OXYCONTIN® 160 MG TABLET IS COMPARABLE TO TWO 80 MG TABLETS WHEN TAKEN ON AN EMPTY STOMACH. WITH A HIGH FAT MEAL, HOWEVER, THERE IS A 25% GREATER PEAK PLASMA CONCENTRATION FOLLOWING ONE 160 MG TABLET. DIETARY CAUTION SHOULD BE TAKEN WHEN PATIENTS ARE INITIALLY TITRATED TO 160 MG TABLETS.
In treating pain it is vital to assess the patient regularly and systematically. Therapy should also be regularly reviewed and adjusted based upon the patient' own reports of pain and side effects and the health professional's clinical judgment.
OxyContin is intended for the management of moderate to severe pain in patients who require treatment with an oral opioid analgesic for more than a few days. The controlled-release nature of the formulation allows it to be effectively administered every 12 hours (see ; PHARMACOKINETICS AND METABOLISM ). While symmetric (same dose AM and PM), around-the-clock, q12h dosing is appropriate for the majority of patients, some patients may benefit from asymmetric (different dose given in AM than in PM) dosing, tailored to their pain pattern. It is usually appropriate to treat a patient with only one opioid for around-the-clock therapy.
It is critical to initiate the dosing regimen for each patient individually, taking into account the patient' prior opioid and non-opioid analgesic treatment. Attention should be given to:
Care should be taken to use low initial doses of OxyContin in patients who are not already opioid tolerant, especially those who are receiving concurrent treatment with muscle relaxants, sedatives, or other CNS active medications (see PRECAUTIONS : Drug-Drug Interactions ).
Patients Not Already Taking Opioids (opioid naive)
Clinical trials have shown that patients may initiate analgesic therapy with OxyContin. A reasonable starting dose for most patients who are opioid naive is 10 mg q12h. If a non-opioid analgesic [aspirin (ASA), acetaminophen (APAP) or a non-steroidal anti-inflammatory (NSAID)] is being provided, it may be continued. If the current non-opioid is discontinued, early upward dose titration may be necessary.
Conversion from Fixed-Ratio Opioid/APAP, ASA, or NSAID Combination Drugs
Patients who are taking 1 to 5 tablets/capsules/caplets per day of a regular strength fixed-combination opioid/non-opioid should be started on 10 to 20 mg OxyContin q12h. For patients taking 6 to 9 tablets/capsules/caplets, a starting dose of 20 to 30 mg q12h is suggested. For those taking 10 to 12 tablets/capsules/caplets a day, 30 to 40 mg q12h should be considered. The non-opioid may be continued as a separate drug. Alternatively, a different non-opioid analgesic may be selected. If the decision is made to discontinue the non-opioid analgesic, consideration should be given to early upward titration.
Patients Currently on Opioid Therapy
If a patient has been receiving opioid-containing medications prior to OxyContin therapy, the total daily (24-hour) dose of the other opioids should be determined.
No fixed conversion ratio is likely to be satisfactory in all patients, especially patients receiving large opioid doses. The recommended doses shown in Table 4 are only a starting point, and close observation and frequent titration are indicated until patients are stable on the new therapy.
|
|||||||||||||||||||||||||||||||||
In all cases, supplemental analgesia (see below) should be made available in the form of immediate-release oral oxycodone or another suitable short-acting analgesic.
OxyContin can be safely used concomitantly with usual doses of non-opioid analgesics and analgesic adjuvants, provided care is taken to select a proper initial dose (see PRECAUTIONS ).
Conversion from Transdermal Fentanyl to OxyContin
Eighteen hours following the removal of the transdermal fentanyl patch, OxyContin treatment can be initiated. Although there has been no systematic assessment of such conversion, a conservative oxycodone dose, approximately 10 mg q12h of OxyContin, should be initially substituted for each 25 µg/hr fentanyl transdermal patch. The patient should be followed closely for early titration as there is very limited clinical experience with this conversion.
Managing Expected Opioid Adverse Experiences
Most patients receiving opioids, especially those who are opioid naive, will experience side effects. Frequently the side effects from OxyContin are transient, but may require evaluation and management. Adverse events such as constipation should be anticipated and treated aggressively and prophylactically with a stimulant laxative and/or stool softener. Patients do not usually become tolerant to the constipating effects of opioids.
Other opioid-related side effects such as sedation and nausea are usually self-limited and often do not persist beyond the first few days. If nausea persists and is unacceptable to the patient, treatment with anti-emetics or other modalities may relieve these symptoms and should be considered.
Patients receiving OxyContin may pass an intact matrix "ghost" in the stool or via colostomy. These ghosts contain little or no residual oxycodone and are of no clinical consequence.
Individualization of Dosage
Once therapy is initiated, pain relief and other opioid effects should be frequently assessed. Patients should be titrated to adequate effect (generally mild or no pain with the regular use of no more than two doses of supplemental analgesia per 24 hours). Rescue medication should be available (see Supplemental Analgesia ). Because steady-state plasma concentrations are approximated within 24 to 36 hours, dosage adjustment may be carried out every 1 to 2 days. It is most appropriate to increase the q12h dose, not the dosing frequency. There is no clinical information on dosing intervals shorter than q12h. As a guideline, except for the increase from 10 mg to 20 mg q12h, the total daily oxycodone dose usually can be increased by 25% to 50% of the current dose at each increase.
If signs of excessive opioid-related adverse experiences are observed, the next dose may be reduced. If this adjustment leads to inadequate analgesia, a supplemental dose of immediate-release oxycodone may be given. Alternatively, non-opioid analgesic adjuvants may be employed. Dose adjustments should be made to obtain an appropriate balance between pain relief and opioid-related adverse experiences.
If significant adverse events occur before the therapeutic goal of mild or no pain is achieved, the events should be treated aggressively. Once adverse events are under control, upward titration should continue to an acceptable level of pain control.
During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between physician, other members of the health-care team, the patient and the caregiver/family.
Special instructions for OxyContin® 80 mg and 160 mg Tablets
(For use in opioid tolerant patients only.)
OxyContin® 80 mg and 160 mg Tablets are for use only in opioid tolerant patients requiring daily oxycodone equivalent dosages of 160 mg or more for the 80 mg tablet and 320 mg or more for the 160 mg tablet. Care should be taken in the prescription of this tablet strength. Patients should be instructed against use by individuals other than the patient for whom it was prescribed, as such inappropriate use may have severe medical consequences.
One OxyContin® 160 mg tablet is comparable to two 80 mg tablets when taken on an empty stomach. With a high fat meal, however, there is a 25% greater peak plasma concentration following one 160 mg tablet. Dietary caution should be taken when patients are initially titrated to 160 mg tablets.
Supplemental Analgesia
Most cancer patients given around-the-clock therapy with controlled-release opioids will need to have immediate-release medication available for "rescue" from breakthrough pain or to prevent pain that occurs predictably during certain patient activities (incident pain).
Rescue medication can be immediate-release oxycodone, either alone or in combination with acetaminophen, aspirin or other NSAIDs as a supplemental analgesic. The supplemental analgesic should be prescribed at 1 / 4 to 1 / 3 of the 12-hour OxyContin dose as shown in Table 5. The rescue medication is dosed as needed for breakthrough pain and administered one hour before anticipated incident pain. If more than two doses of rescue medication are needed within 24 hours, the dose of OxyContin should be titrated upward. Caregivers and patients using prn rescue analgesia in combination with around-the-clock opioids should be advised to report incidents of breakthrough pain to the physician managing the patient's analgesia (see Information for Patients/Caregivers ).
|
The intent of the titration period is to establish a patient-specific q12h dose that will maintain adequate analgesia with acceptable side effects for as long as pain relief is necessary. Should pain recur then the dose can be incrementally increased to re-establish pain control. The method of therapy adjustment outlined above should be employed to re-establish pain control.
During chronic therapy, especially for non-cancer pain syndromes, the continued need for around-the-clock opioid therapy should be reassessed periodically (e.g., every 6 to 12 months) as appropriate.
Cessation of Therapy
When the patient no longer requires therapy with OxyContin tablets, patients receiving doses of 20-60 mg/day can usually have the therapy stopped abruptly without incident. However, higher doses should be tapered over several days to prevent signs and symptoms of withdrawal in the physically dependent patient. The daily dose should be reduced by approximately 50% for the first two days and then reduced by 25% every two days thereafter until the total dose reaches the dose recommended for opioid naive patients (10 or 20 mg q12h). Therapy can then be discontinued.
If signs of withdrawal appear, tapering should be stopped. The dose should be slightly increased until the signs and symptoms of opioid withdrawal disappear. Tapering should then begin again but with longer periods of time between each dose reduction.
Conversion from OxyContin to Parenteral Opioids
To avoid overdose, conservative dose conversion ratios should be followed. Initiate treatment with about 50% of the estimated equianalgesic daily dose of parenteral opioid divided into suitable individual doses based on the appropriate dosing interval, and titrate based upon the patient' response.
OxyContin® (oxycodone hydrochloride controlled-release) tablets are solid dosage forms that pose no known health risk to health-care providers beyond that of any controlled substance. As with all such drugs, care should be taken to prevent diversion or abuse by proper handling.
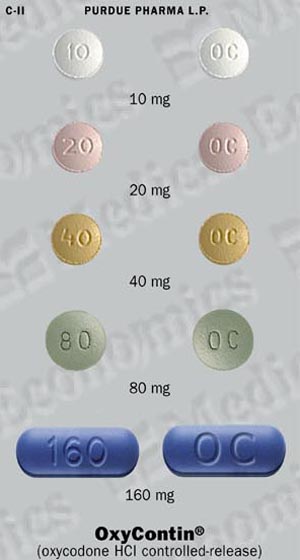
OxyContin® (oxycodone hydrochloride controlled-release) 10 mg tablets are round, unscored, white-colored, convex tablets bearing the symbol OC on one side and 10 on the other. They are supplied as follows:
NDC 59011-100-10: child-resistant closure, opaque plastic bottles of 100
NDC 59011-100-25: unit dose packaging with 25 individually numbered tablets per card; one card per glue end carton
OxyContin® (oxycodone hydrochloride controlled-release) 20 mg tablets are round, unscored, pink-colored, convex tablets bearing the symbol OC on one side and 20 on the other. They are supplied as follows:
NDC 59011-103-10: child-resistant closure, opaque plastic bottles of 100
NDC 59011-103-25: unit dose packaging with 25 individually numbered tablets per card; one card per glue end carton
OxyContin® (oxycodone hydrochloride controlled-release) 40 mg tablets are round, unscored, yellow-colored, convex tablets bearing the symbol OC on one side and 40 on the other. They are supplied as follows:
NDC 59011-105-10: child-resistant closure, opaque plastic bottles of 100
NDC 59011-105-25: unit dose packaging with 25 individually numbered tablets per card; one card per glue end carton
OxyContin® (oxycodone hydrochloride controlled-release) 80 mg tablets are round, unscored, green-colored, convex tablets bearing the symbol OC on one side and 80 on the other. They are supplied as follows:
NDC 59011-107-10: child-resistant closure, opaque plastic bottles of 100
NDC 59011-107-25: unit dose packaging with 25 individually numbered tablets per card; one card per glue end carton
OxyContin® (oxycodone hydrochloride controlled-release) 160 mg tablets are modified caplet-shaped, unscored, blue-colored, convex tablets bearing the symbol OC on one side and 160 on the other. They are supplied as follows:
NDC 59011-109-10: child-resistant closure, opaque plastic bottles of 100
NDC 59011-109-25: unit dose packaging with 25 individually numbered tablets per card; one card per glue end carton
Store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F).
Dispense in tight, light-resistant container.
CAUTION
DEA Order Form Required.
Manufactured by The PF Laboratories, Inc.
Totowa, N.J. 07512
Distributed by Purdue Pharma L.P.
Stamford, CT 06901-3431
Copyright© 1995, 2000 Purdue Pharma L.P.
U.S. Patent Numbers 4,861,598; 4,970,075; 5,266,331; 5,508,042; 5,549,912; and 5,656,295
June 6, 2000 M4909 00PO10
 |